Take home message
Milk enzymes change differently when heated. Graphically it can be made clear that each enzyme has its own time and temperature relationship. This makes it clear which enzymes are more and less sensitive to heating.
Denaturation and Efficacy
Many enzymes are present in milk. Blanc (1982) mentions 60 different enzymes. In dairy technology enzymes are used as an indication of technological process change, such as heat and pressure. Intact enzymes can be of benefit, if they support digestion and contribute to the defense against unwanted bacteria. Milk fat consists for the most part of triglycerides, made up of glycerol, to which three fatty acids are linked. Lipase in milk, like human lipase produced in our pancreas, is responsible for splitting the triglycerides in the small intestine. Lipase breaks down the fatty acids from the glycerol, making the fatty acids available for further digestion. Milk lipase causes rancidity and pasteurization of milk inactivates the milk lipase. Enzyme concentrations can vary widely between cow milk and human milk. For example, the concentration of Lactoperoxidase (LPO) is about 100 times higher and Xanthinoxidase (XO) is 10 times higher in cow milk compared to human milk. Lipase and Catalase, on the other hand, are 10 times lower in cow milk. LPO together with Lysozyme contribute to the anti-bacterial activity of raw milk, in other words there is an inhibition of bacterial development such as Escherichia coli and Staphyloccus aureus. When our mouth saliva comes into contact with XO, hydrogen peroxide (H2O2) is created, which in turn suppresses the growth of pathogenic bacteria such as S. aureus and Salmonella enterica (Ozturk et al., 2019). The enzymes are thus associated with the suppression of unwanted growth of pathogenic bacteria. In order to take advantage of this mechanism, it is important that the enzymes are still active. There is 95% denaturation of XOin UHT milk, and S. aureus can therein grow to more than 10 million bacteria after inoculation in the UHT milk. S. aureus does not grow in raw milk and pasteurized milk, as the enzyme system is still intact.
Heating
Enzymes are different when it comes to their susceptivity to denaturation. Heating can change the structure of the molecule, thereby causing the enzyme to lose its effect. Some are inactivated already at low temperatures, others only at higher temperatures. However, it is always about the combination of time and temperature. Figure 1 shows, on the basis of the heating temperature (horizontal) and the heating time (vertical semi-logarithmic), where the various enzymes are inactived. Alkaline Phosphatase (ALP) is shown in red. ALP is the enzyme used in industry to demonstrate that milk is pasteurized. The two points at the end of the line indicate two different forms of pasteurization: the batch pasteurization involves heating at 62-63oC for 30 minutes, the HTST (High Temperature Short Time) is based on a continuous pasteurizer, where the milk is kept at 72oC for 15 seconds.
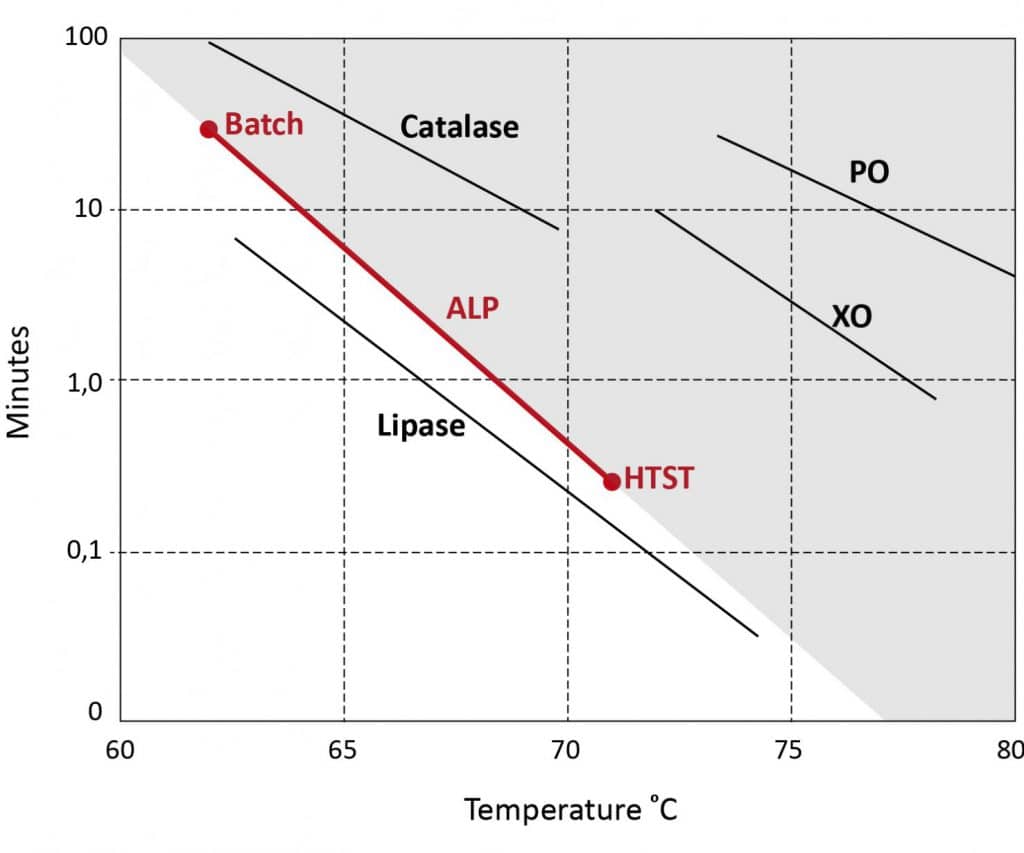
Everything to the left of the ALP line in the white part is simultaneously rendered inactive by pasteurization, such as the enzyme lipase. Enzymes that lie to the right of the ALP line in the shaded area have not yet or have not been deactivated completely. To do this, the milk must be pasteurized longer, or higher temperatures must be used.
The choice for these combinations of pasteurization temperature and time stems from the killing of some unwanted bacteria that were regularly found in raw milk at the beginning of the 20th century, namely those that caused tuberculosis and brucellosis. The Tuberculosis bacteria (TB) are killed at combinations of time and temperature that would correspond to a line slightly to the left of the ALP line (not shown). This means that milk that has been pasteurized will not contain live TB.
In European countries, UHT milk is frequently and sometimes exclusively consumed. This milk is heated very briefly at a temperature of 135-150oC (a few seconds). This not only kills the bacteria, but also prevents various bacterial spores from developing. In this heating range almost all enzymes from the grey area have also become largely ineffective. A subsequent article will examine research into real forms of heating, namely HTST and UHT, and the consequences for enzymes, bacterial growth and protein change.
This article is based on B.Blanc (1982). Les protéines du lait à activité enzymatique et hormonale. Le Lait (1982), 62, 350-395




